Stem: Hard swelling in right inguinal region , GP sent her for biopsy.(Malignant Melanoma Mets)
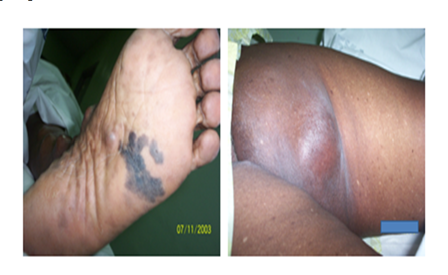
DD ?
Differential diagnoses to consider with inguinal lymphadenopathy:
1. Infection:
– Cellulitis of lower limbs: Reactive inflammatory lymphadenitis secondary to bacterial infection of legs/feet. Nodes tender, enlarged but mobile. Infection and inflammation elsewhere need exclusion.
– Venereal infections: Syphilis, chancroid, herpes, lymphogranuloma venereum (LGV) can all cause inguinal lymphadenopathy. Sexual history and serology needed to determine diagnosis. Nodes often matted, sensitive.
– Lymphogranuloma venereum: From C. trachomatis, causes large buboes with overlying edema and erythema. Often in MSM population with high risk behaviors. Needs antibiotic treatment.
2. Malignancy:
– Metastatic melanoma: Most likely with known lower extremity or trunk primary. Nodes often hard, non-tender, rapidly growing. Urgent biopsy needed for diagnosis and staging.
– Lymphoma: Especially Hodgkin’s lymphoma. Nodes non-tender but rubbery, often with loss of fat pads in area. Excisional biopsy, histology, IHC and possible molecular studies required to classify lymphoma and determine optimal treatment.
– Metastatic squamous cell carcinoma: Can arise from anogenital area, especially in elderly or immunosuppressed patients. Biopsy needed to locate primary site and develop a comprehensive treatment plan. Nodes often stony hard and fixed.
3. Other:
– Sarcoidosis: Can present with bilateral hilar and inguinal lymphadenopathy. Non-tender but enlarged nodes. Biopsy showing non-caseating granulomas, +/- raised ACE levels. Exclusion of malignancy always needed.
– HIV infection: Generalized lymphadenopathy may be a presenting sign, especially with opportunistic infections. Anti-HIV serology, culture and biopsy as indicated based on associated symptoms.
– Toxoplasmosis: May cause painful inguinal lymphadenitis, especially in immunocompromised patients. Exposure to cats, positive Toxo titers. Treatment with anti-Toxo medications.
In summary, there are many possibilities to consider with inguinal lymph node enlargement. A thorough history including sexual history and exposures, physical exam assessing nodes and primary sites, and appropriate investigations including biopsy, culture, serology and imaging are needed to determine the correct diagnosis and optimize treatment in each patient. Malignancy must always be ruled out urgently, even when infection seems a likely possibility. Prompt diagnosis and management is key.
Q. Types of MM ? Depending on location, shape and growth outward/ downward into dermis
The major subtypes of malignant melanomas are:
1. Lentigo maligna melanoma: Arises from lentigo maligna, a slow-growing melanoma in-situ. Found on chronically sun-damaged skin, especially faces of elderly. Grows slowly outwards before becoming invasive. Prognosis generally good with early excision.
2. Superficial spreading melanoma: Most common type. Initially grows outwards, irregularly shaped with varied pigmentation. Becomes invasive and metastasizes. Excision when <1mm thick offers good prognosis.
3. Nodular melanoma: Rapidly growing dark nodule, often with bleeding and ulceration. Highly invasive, metastatic and aggressive. Poor prognosis, requires urgent wide excision.
4. Acral lentiginous melanoma: On palms, soles and nail beds. Often non-pigmented or variably colored, irregularly shaped. Locally invasive and metastasizes. Can be difficult to diagnose, requiring high index of suspicion. Generally poor prognosis.
5. Desmoplastic melanoma: Rare, often amelanotic. Found on sun-exposed areas, invasive with tendency to recur locally. Difficult to diagnose and treat, with high risk of metastasis. Requires aggressive management.
Other less common types:
-Mucosal melanoma: On genital and oral mucous membranes. Pigmented or non-pigmented, invasive and metastasizes readily. Diagnosis often delayed, poor prognosis.
-Uveal melanoma: Within the uvea of the eye – iris, ciliary body and choroid. Detected on routine eye exam. Treatment with surgery, radiation and chemo. Metastasis to liver has poor prognosis.
Within these subtypes, key factors determining prognosis are:
1. Tumor thickness – Measured in Breslow’s depth. <1mm has 95% 10 year survival, >4mm has 50% 5 year survival.
2. Ulceration: Breach of epidermis over tumor. Decreases survival by 50% compared to non-ulcerated tumors of same thickness.
3. Mitotic rate: Number of actively dividing cells per mm2 of tumor. Higher mitotic rate indicates more aggressive behavior.
4. Tumor staging – TxNxCx based on thickness, ulceration, lymph node involvment and metastatic spread. Higher stage = worse prognosis.
5. Metastasis – Draining lymph node, in-transit, distant skin, subcutaneous, visceral, all decrease overall survival significantly.
6. Location – Head and neck best, trunk intermediate, extremities worst prognosis.
Treatment of all melanomas involves surgical excision, with margins dictated by stage. Adjuvant treatments like immunotherapy, targeted therapy or radiation may also be used based on risk factors and location. Close follow up with skin exams, lymph node observation and regular imaging is required long term based on patient’s risk.
Q. What is Epitheloid Melanoma ?
Malignant melanoma cells can have two main morphologies:
1. Epithelioid cells: Round to polygonal cells with abundant cytoplasm, large vesicular nuclei and prominent nucleoli. Typified by the ‘fried egg’ appearance. These cells grow in sheets and nests.
2. Spindle cells: Elongated, fusiform cells with blunt-ended nuclei. Spindle cells grow in fascicles with a storiform or whorled pattern.
Epithelioid cell melanomas, specifically epithelioid cell predominant melanomas, refer to melanomas composed primarily or exclusively of epithelioid malignant melanoma cells. These tend to exhibit the following features:
1. Diffuse sheet-like growth: Epithelioid cells grow in cohesive sheets and broad nests, lacking the fascicular pattern of spindle cell tumors.
2. Abundant cytoplasm: The polygonal epithelioid cells have abundant eosinophilic cytoplasm surrounding large round to oval nuclei.
3. Prominent nucleoli: The epithelioid cell nuclei display large eosinophilic nucleoli, some with a ‘fried egg’ appearance. This is in contrast to the small inconspicuous nucleoli of spindle cell and desmoplastic melanomas.
4. Brisk mitotic rate: There are numerous mitotic figures throughout the tumor, indicating rapid proliferation.
5. Vascular invasion: These tumors often show infiltration of vascular spaces by epithelioid melanoma cells, indicating high metastatic potential.
Epithelioid cell predominant melanomas tend to be highly aggressive, with greater risk of recurrence, metastasis and mortality compared to spindle cell melanomas. Prognosis correlates with other features like mitotic rate, ulceration, tumor thickness, etc. Treatment, as with other melanomas, involves wide local excision of the primary tumor based on stage. Epithelioid morphology is considered high risk, so close follow up and consideration of adjuvant treatment may be warranted.
These tumors can also be difficult to distinguish from carcinoma (especially renal cell carcinoma) and epithelioid sarcoma, so immunohistochemistry is often required to confirm the diagnosis of malignant melanoma. S-100, HMB-45, Melan-A and Sox10 are positive in melanoma, while negative for cytokeratins. A diagnosis of epithelioid cell melanoma, especially when poorly differentiated, often requires expert dermatopathological review.
Q. Where to Examine this lady?
For this patient presenting with suspected metastatic melanoma to inguinal lymph nodes, a full body exam and staging workup should be performed to determine appropriate management.
Areas to examine include:
1. Primary melanoma site: Given the location of inguinal nodal metastasis, the primary tumor likely arose on the lower extremities (legs, hips, buttocks), trunk (lower abdomen, perineum) or genitalia (vulva, vagina). A careful skin exam looking for suspicious pigmented lesions, especially on glabrous skin of nail beds, soles and genitalia. Any suspicious lesions should be biopsied.
2. Regional lymph nodes: In addition to the enlarged right inguinal node, examine for other palpable inguinal, femoral or ilio-obturator lymph nodes which may also harbor metastasis. Note size, consistency, mobility and overlying skin changes of any palpable nodes.
3. Chest: Examine lungs for signs of effusion (dullness to percussion, reduced breath sounds) or masses. Check for palpable supraclavicular lymph nodes which may indicate metastatic spread to mediastinal nodes.
4. Abdomen: Examine for hepatomegaly, splenomegaly or abdominal masses which could indicate metastasis to liver, peritoneum or retroperitoneum. Check umbilicus and periumbilical area for metastatic nodules (Sister Mary Joseph nodules).
5. Skin and subcutis: Carefully examine skin and palpate subcutaneous tissues over chest, abdomen, back and flanks for any suspicious nodules which may be in-transit or distant cutaneous metastases.
Based on findings from history, exam and initial biopsy, further staging workup should include:
1. CT chest, abdomen and pelvis: To evaluate for visceral and pelvic metastasis not detected on physical exam. Also assesses pelvic nodes which may be metastatic from lower extremity or genital primary melanoma.
2. PET/CT scan: Provides whole body visualization to search for other metabolically active primary tumors, nodal disease or distant metastatic spread. Guides biopsy of any abnormal radiotracer uptake.
3. MRI brain: Though patient currently asymptomatic, obtain baseline MRI brain given risk of metastasis, especially with epithelioid morphology or inguinal node involvement.
4. Serum LDH: As a marker for tumor burden, elevated LDH may reflect increased metastatic spread or higher stage disease.
5. Consider SLNB if primary site located: Provides pathological staging of nodes even if clinically negative. Biopsies any ‘hot spot’ on lymphoscintigraphy for analysis and risk stratification.
The results of the full staging workup will determine appropriate multidisciplinary management, such as referral to medical oncology, radiation oncology, and other specialties as needed based on sites of metastasis and options for systemic treatment if stage IV disease. Prognosis ultimately depends on ability to cutaneously map and resect the primary tumor, level of nodal and distant spread, and any response to adjuvant therapies. Close surveillance will also be required given high risk of further metastatic disease over follow up.
Q. Treatment of this lady?
For this patient with metastatic melanoma to inguinal lymph nodes, the treatment approach should be:
1. Wide local excision of the primary tumor: Once the primary melanoma site has been identified on skin exam and/or imaging, it should be excised with appropriate margins based on Breslow thickness. Wider margins, down to the fascia, may be needed for suspected deeper or high-risk lesions. Excision provides local control and complete staging/prognostic information.
2. Completion inguinal lymph node dissection (ILND): Given the patient now has proven metastatic inguinal nodal involvement, surgical resection through lymph node dissection (rather than just sentinel node biopsy) is recommended to remove any further positive nodes and reduce risk of disease progression/recurrence in those basins.
3. Radiation therapy: The groin basin should receive adjuvant radiotherapy after ILND to eliminate any remaining microscopic disease not removed surgically. Lymph node dissection presents high risk margins and radiotherapy at 4-6 weeks post-op helps maximize locoregional control.
4. Systemic therapy: Even after local control is achieved, metastatic melanoma at presentation indicates a high likelihood of additional occult spread elsewhere. Systemic therapy options should be considered to target circulating disease cells and prevent further spread and metastasis. Options include:
– Immunotherapy like anti-PD1 inhibitors: First-line immunotherapy has significant impact on survival in stage IV melanoma. Given patient’s young age, immunotherapy offers potentially long-lasting responses with manageable side effect profile.
– Targeted therapy for BRAF+ melanoma: If oncogenic BRAF mutation is detected, targeted BRAF and MEK inhibitors can be very effective, at least as initial treatment, with quick responses. However, resistance often develops.
– Chemotherapy: Limited responses to dacarbazine or temozolomide. Mainly used for immunotherapy/targeted therapy refractory disease. Toxicity can be significant for limited benefit.
-Biologic therapy: Interferon alfa or interleukin-2 were used historically, now mainly for high-dose bolus IL-2 responsive disease. Limited applications with toxicities.
– Clinical trial: Given poor prognosis with metastatic disease at onset, eligible patients should be offered clinical trial enrollment for promising new therapies. Many novel immunotherapy and targeted combination regimens are in development.
5. Close monitoring: Will require lifelong follow up with 3-6 monthly physical exams, lymph node assessment, and regular cross-sectional imaging to monitor for disease progression or recurrence. Early detection of additional metastases or recurrent nodal disease will guide potential for additional surgical resection or modification of systemic therapy as needed.
The prognosis for this level of disease at presentation is often guarded to poor, with median survivals of 6-9 months if left untreated, but with modern therapies and close surveillance, some patients are achieving prolonged survival and remission. The patient’s response to initial treatments and absence of rapid disease progression will provide some sense of likely clinical course and outcomes over the next 2-3 years.
Q. How to know the phenotype of tumor?
Immunohistochemistry (IHC) is a key technique used to determine the phenotype of a tumor. It involves staining tumor tissue sections with antibodies targeted against specific proteins to determine if they are expressed or not. The binding of the antibodies can then be visualized under the microscope using a chromogenic substrate or fluorescence.
Some of the main uses of IHC in tumor diagnosis and determining phenotype include:
1. Confirming the histological diagnosis: Stains for proteins like cytokeratins, S100, HMB45 and Melan-A are used to confirm if a tumor is epithelial, melanoma or other lineage. This helps classify the tumor properly for treatment.
2. Determining origin: Stains for organ-specific markers like TTF-1 (lungs), PAX-8 (gynecologic), and CDX-2 (gastrointestinal) help determine the origin of a metastatic tumor with unknown primary site. This guides imaging, biopsy and treatment needed.
3. Identifying predictive markers: Estrogen receptor (ER), progesterone receptor (PR) and HER2 status in breast cancer determines if targeted hormone or trastuzumab therapy may be options. Similar markers in other cancers help guide precision treatment.
4. Providing prognostic information: Ki-67, a proliferative marker, helps determine the aggressiveness of some cancers. Neuroendocrine markers in prostate cancer indicate more aggressive behavior.
5. Distinguishing between carcinomas, sarcomas and melanomas: A panel of stains (eg. cytokeratins, S100, desmin) help classify undifferentiated tumors as epithelial, melanocytic or mesenchymal in origin. This guides appropriate management.
6. Detecting spread to lymph nodes: Stains for markers like CK19, mammaglobin or prostatic acid phosphatase (PAP) help determine if tumor cells present in lymph nodes are metastatic spread from an epithelial primary like breast, colon or prostate cancer. This provides important staging information to guide treatment.
7. Researching new markers: IHC allows for screening of expression of potential new predictive, prognostic or therapeutic markers that may shed light on tumor behavior or identify new drug targets. Selected cases can then undergo molecular analysis.
Therefore, IHC provides a rapid, cost-effective method for determining tumor phenotype, including histological diagnosis and subtype, origin, markers of prognosis/prediction and spread. A panel of stains is often required to obtain this full range of information for cancer diagnosis, staging and management. Molecular studies and RNA/DNA analysis provide another, more detailed level of information to further understand tumor biology and personalize treatment.
Q. Post operative wound red, swollen , culture revealed diplococci, Examples ?
There are several possibilities to consider with a postoperative wound infection that grows gram positive diplococci on culture:
Gram positive diplococci:
1. Streptococcus pneumoniae: A common cause of surgical site infections, especially in elderly or immunocompromised patients. Sensitive to penicillins, cephalosporins, fluoroquinolones. Can cause severe disease like sepsis if not properly treated.
2. Enterococcus species: Especially Enterococcus faecalis, part of normal gut flora that can contaminate wounds. Usually sensitive to ampicillin, vancomycin, linezolid and daptomycin depending on species. Require prolonged antibiotic course to clear.
3. Streptococcus pyogenes: Beta-hemolytic strep, the causative agent of strep throat and scarlet fever. Can rarely cause severe soft tissue infections and necrotizing fasciitis if enters surgical wounds. Sensitive to penicillins and cephalosporins.
Gram negative diplococci:
1. Neisseria meningitidis or Neisseria gonorrhoeae: Much less likely in a surgical site infection but possible from contamination with nasopharyngeal or genital flora. Require isolation and growth on selective media. Cause severe disease if in the bloodstream but rare in wounds. Treated with ceftriaxone, ciprofloxacin or other antipseudomonal agents.
2. Moraxella catarrhalis: Another unusual cause of wound infections. Part of respiratory flora, tends to affect those with underlying lung disease. Sensitive to macrolides, tetracyclines, trimethoprim-sulfamethoxazole.
Given the description, the most likely organisms are Strep. pneumoniae, Enterococcus species and possibly Strep. pyogenes – all of which are routinely sensitive to cephalosorins like cefazolin, the prophylactic agent commonly used in surgery. Therefore, a cephalosporin alone may be adequate for uncomplicated infection. However, broader coverage may be needed in some situations:
– Severe or rapidly progressive infection: May add vancomycin, linezolid or daptomycin for suspected MRSA or VRE.
-Immunocompromised patient: Consider adding coverage for Pseudomonas (piperacillin-tazobactam, ciprofloxacin) and anaerobes (metronidazole) which can contaminate wounds.
-Lack of improvement in 2-3 days: May need to broaden to cover gram negatives like species of Enterobacterales (ESBL-producers) in addition to adding MRSA/VRE coverage.
-Signs of sepsis or osteomyelitis: Requires urgent direct culture of infected fluid or bone, and targeted broad-spectrum IV antibiotics to cover all likely pathogens.
The key is appropriate early cultures to determine the causative organism(s) and antibiotic sensitivity profile so that targeted therapy can be initiated, and initial broad coverage narrowed. All postsurgical wound infections require close monitoring and potential surgical debridement to adequately clear the infection.
Q. Toxemia with rapidly spreading infection?
Necrotizing fasciitis is a serious, rapidly progressive soft tissue infection that involves the fascia and subcutaneous tissues. It is characterized by:
1. Severe pain: Disproportionate to visible skin changes early on. Pain often described as throbbing or stabbing, exceeding the apparent cellulitis.
2. Rapid spread: Infection spreads very quickly along the fascial plane, advancing up to 1 inch per hour. Skin changes lag behind the advancing edge of infection.
3. Systemic toxicity: Patients appear severely ill, with high fevers, tachycardia, hypotension, and evidence of sepsis or septic shock (organ dysfunction). This results from a massive bacterial toxin release into the circulation.
4. Bullae and skin necrosis: As infection advances, hemorrhagic bullae (fluid-filled blisters) form and skin develops a dusky, necrotic appearance with areas of purpura (bruising) or gangrene. Skin can develop a ‘peau d’orange’ texture.
5. Crepitus: A crackling sensation may be felt in the subcutaneous tissues due to gas production by bacteria like Group A Strep, Clostridium perfringens or anaerobic bacteria. This is a surgical emergency.
6. Diagnosis: Made primarily on clinical grounds based on appearance and rapid progression. Lab tests show severe inflammation and toxic changes. X-rays or CT may show gas in soft tissues. Cultures and tissue biopsies are obtained in OR during debridement.
Treatment involves:
1. Emergent and aggressive surgical debridement of necrotic tissues – repeated until healthy bleeding tissues are reached. This is the primary life-saving intervention.
2. IV antibiotics: Broad coverage for pathogens like MRSA, Group A Strep, anaerobes and Gram negatives. Penicillin, clindamycin, vancomycin and carbapenems are often used, then tailored based on culture results.
3. Critical care: Patients often require ICU management for wound care, ventilation, vasopressors, fluid/electrolyte management due to the severity of their condition.
4. Hyperbaric oxygen: Used as an adjunct in some cases to help healthy tissue regeneration and infection control.
5. Repeat debridements: Required every 1-2 days until infection stops advancing and a healthy wound bed is achieved. Reconstruction of any tissue defects then follows once infection has cleared.
Mortality can exceed 30% even with treatment, especially if diagnosis or surgical debridement is delayed beyond 24-48 hours after onset of symptoms. All physicians must maintain a high index of suspicion for this surgical emergency to provide the best opportunity for stopping its progression and saving the patient’s life and limb.
Q. What is SIRS ?
SIRS refers to a systemic activation of the inflammatory response, the body’s reaction to an infectious or non-infectious insult. It is characterized by two or more of the following criteria:
1. Abnormal body temperature: Fever (>38°C or 100.4°F) or hypothermia (<36°C or 96.8°F)
2. Tachycardia: Heart rate >90 beats/minute
3. Tachypnea: Respiratory rate >20 breaths/minute OR PaCO2 <32 mmHg
4. Abnormal white blood cell count: >12,000/mm3 or <4,000/mm3, or >10% band forms (immature granulocytes)
5. Hyperglycemia: Plasma glucose >120 mg/dL in the absence of diabetes mellitus
6. Altered mental status: Confusion, lethargy or agitation
The pathophysiology of SIRS involves a complex interplay between pro-inflammatory and anti-inflammatory mediators known as cytokines. An infectious process, trauma, burn, pancreatitis or other insult triggers an initial pro-inflammatory response. This then leads to a cascade of immunologic reactions that can become self-sustaining, resulting in the signs of systemic inflammation we recognize as SIRS.
Some key complications of SIRS include:
1. Sepsis: SIRS due to suspected or confirmed infection. Associated with severe morbidity and mortality.
2. Septic shock: Sepsis associated with tissue hypoperfusion/organ dysfunction from vasodilation and capillary leak. Medical emergency.
3. Multiple Organ Dysfunction Syndrome (MODS): Malfunction of two or more organ systems as a result of a dysregulated inflammatory response. Can be fatal if severe or prolonged.
4. Anasarca/Capillary leak: Massive whole body edema from loss of capillary integrity. Requires ICU level supportive care.
Treatment of SIRS involves:
1. Treating the underlying trigger: Antibiotics for infection, drainage/debridement of abscess, treatment of pancreatitis, etc.
2. Early goal-directed therapy: IV fluids, vasopressors, oxygen, ventilation support as needed. Aimed at organ perfusion and oxygenation.
3. ICU level monitoring and care: To monitor for and promptly treat complications like sepsis, shock or organ dysfunction.
4. Low dose steroids: Controversial, as may help switch off uncontrolled inflammation but also inhibit immune response. Limited evidence for routine use.
5. Other therapies: Aimed at balancing the inflammatory response, including: NSAIDs, immunoglobulins, cytokine antagonists, etc. Still largely experimental.
Q. What happens to lung in SIRS?
One of the major complications of Systemic Inflammatory Response Syndrome (SIRS) and sepsis is Acute Respiratory Distress Syndrome (ARDS). This refers to acute inflammatory lung injury that results in impaired gas exchange and decreased lung compliance.
In ARDS, the initial inflammatory response triggers an influx of neutrophils and fluid into the lungs. This results in:
1. Alveolar damage: The air sacs (alveoli) become flooded with proteinaceous fluid and debris, damaging type I alveolar cells required for gas exchange. Surfactant production is also impaired.
2. Capillary leak: Increased permeability of lung capillaries allows large amounts of fluid and proteins to move into the interstitium and alveoli. This causes pulmonary edema and reduced blood flow/oxygenation.
3. Neutrophilic infiltration: Massive numbers of neutrophils migrate into the lungs, releasing proteases, cytokines and reactive oxygen species that damage lung tissue and impair function.
4. Diffuse alveolar damage: Widespread damage to alveolar walls and microvasculature. Alveoli become filled with hyaline membranes, fibrin and cellular debris.
This process results in progressive respiratory failure:
– Decreased lung compliance: Stiff, poorly-expanded lungs with reduced ventilation
– Impaired gas exchange: Poor matching of ventilation to perfusion prevents adequate oxygenation of blood and removal of carbon dioxide.
– Hypoxemia/Hypercarbia: pO2 drops while pCO2 rises, requiring increasing oxygen/ventilator support.
– Bilateral infiltrates on CXR: Typical ‘white-out’ or diffuse hazy infiltrates representing edema and inflammation.
Diagnosis is based on acute onset of respiratory failure with bilateral pulmonary infiltrates and hypoxemia not explained by heart failure or other condition. Comparison of arterial blood gas to calculated expected pO2 based on delivered oxygen concentration (PaO2/FiO2 ratio) helps grade severity.
Treatment focuses on:
1. Lung-protective ventilation: Low tidal volumes (4-6 mL/kg), permissive hypercapnia, PEEP, prone positioning. Aims to minimize ventilator-induced lung injury.
2. Fluid restriction: To reduce edema. Diuretics are also often used.
3. Treatment of underlying SIRS/Sepsis: With antibiotics, source control, ICU monitoring/treatment of shock and organ dysfunction.
4. Steroids: Role controversial. May be used in refractory hypoxemia unresponsive to other measures based on risk-benefit in individual patients.
5. Prone positioning: Shifts fluid distribution to improve oxygenation. Requires specialized beds and staffing.
6. ECMO: Used in severe cases to provide oxygenation/ventilation support when lungs are unable to do so, even with protective ventilation. Allows lungs to rest and potentially recover function.
Q. Define and explain pathophysiology of ards ARDS?
Acute Respiratory Distress Syndrome (ARDS) is a syndrome of acute inflammatory lung injury characterized by:
1. Diffuse alveolar damage: Widespread damage to the air sacs (alveoli) and capillaries of the lung. The alveolar epithelial and endothelial cells are primarily affected. This impairs gas exchange and reduces lung compliance.
2. Pulmonary edema and increased capillary permeability: Damage to the capillary endothelium allows large amounts of fluid and proteins to leak into the interstitium and alveoli. This causes stiff, heavy lungs with impaired blood flow and oxygenation.
3. Surfactant dysfunction: Inactivation of surfactant reduces surface tension in the alveoli, allowing them to collapse and impairing their stability. This worsens gas exchange and work of breathing.
4. Activation of innate immunity: Massive neutrophil invasion and cytokine/mediator release causes acute inflammation in the lungs. This propagation of inflammation results in a cycle of ongoing damage.
5. Abnormal Coagulation: Intra-alveolar fibrin deposition occurs due to activation of clotting systems. This fibrin also impairs gas diffusion and lung function.
The pathophysiology of Acute Respiratory Distress Syndrome (ARDS) involves a complex series of events:
1. Initial insult: Direct (pneumonia, aspiration) or indirect (sepsis, trauma) lung injury leads to damage of the alveolar-capillary membrane. This activates an inflammatory response in the lungs.
2. Inflammation: Inflammatory mediators like cytokines, chemokines, proteases, and reactive oxygen species are released, attracting neutrophils into the lungs. These mediators and cells cause further damage to the alveoli and pulmonary capillaries. A vicious cycle develops that propagates inflammation.
3. Capillary leak: Inflammation and endothelial injury increase the permeability of lung capillaries. This allows protein-rich fluid to leak into the interstitium and alveolar sacs, causing pulmonary edema that inhibits gas exchange.
4. Surfactant dysfunction: Alveolar type II cells responsible for producing pulmonary surfactant are damaged and surfactant is inactivated. This reduces surface tension in the alveoli allowing them to collapse, especially on expiration. Lung compliance decreases and work of breathing increases.
5. Alveolar-capillary membrane damage: Widespread damage occurs to both the alveolar epithelial cells (type I) and the capillary endothelial cells. This destroys the barrier between the airspaces and the pulmonary capillaries, filling alveoli with fluid and cells. Gas diffusion is severely impaired.
6. Fibrin deposition: Damage to the capillaries activates clotting cascades, causing fibrin clots to form within alveolar spaces and microvasculature. This further obstructs gas exchange and impedes lung function, while impairing surfactant activity.
7. Atelectasis: Loss of surfactant and alveolar edema/fluid causes alveoli to collapse (atelectasis), with poor inflation even under high pressures. Much of the lung is poorly aerated or non-aerated, limiting ventilation and exacerbating shunting.
8. Ventilation-perfusion mismatch: With areas of atelectasis adjacent to overinflated alveoli, matching ventilation and perfusion in the lungs becomes impossible. Significant shunting of deoxygenated blood limits arterial oxygenation despite high inspired oxygen.
This acute inflammatory lung injury takes days to weeks to resolve as the stimuli and propagation of inflammation are gradually controlled, alveolar fluid is reabsorbed, and repair of the damaged epithelium occurs. Supportive care in the ICU is aimed at limiting further damage while facilitating the body’s inherent mechanisms of resolution and healing.
In summary, widespread alveolo-capillary damage along with surfactant inactivation, inflammation, edema and intra-alveolar fibrin all contribute to the respiratory failure seen in ARDS. Supportive care aims to minimize ongoing injury while allowing the affected tissues opportunities to heal.


